Ferriprox®
-

Product Information
Scientific name: Deferiprone
Brand name: Ferriprox®
RESPONSIBLE: Disc Medicine Inc and Mabwell Therapeutics -

Clinical Trial/Study Information
Trial Name: N/A
Code: N/A (Τo be initiated in second half of 2023)
Phase: 1 (Proof-of Mechanism)
Eligible patient diagnosis: Healthy Volunteers
No. of Patients enrolled: N/A
Study Sites: N/A Sites per countryAnticipated completion date: 2024
Scope of the Study / Aim: Targets TMPRSS6 with an antibody to enable the increase of hepcidin and reduction of iron -

Regulatory Information
Status: Not Authorised
Additional notable points:
- FDA: Received acceptance of an Investigational New Drug (IND) application from the U.S. Food and Drug Administration (FDA) in November 2022.
Update: 22 December 2025
No update available.
Update: 30 September 2025
No update available.
Update: 30 June 2025
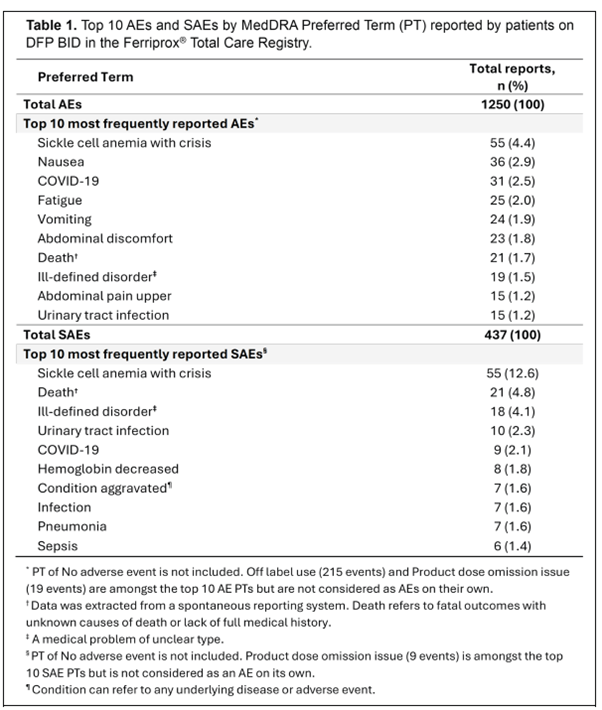
Data presented at the 30th EHA Annual Congress (12 – 15 June) in Milan, Italy, showed the real-world safety profile for deferiprone twice a day (BID) from 425 patients, children, and adults, including 133 with thalassaemia and 197 with SCD, in the USA.
The most frequent adverse events are shown in the table below:
Update: 31 March 2025
No update available.
Update: 19 December 2024
No update available.
Update: 30 September 2024
No update available.
Update: 30 June 2024
No update available.
Update: 31 March 2024
No update available.
Update: 20 December 2023
No update available.
Update: 30 September 2023
No update available.
Update: 30 June 2023
Health Canada has approved Ferriprox® extended-release (twice daily) tablets (1,000mg) for patients with transfusional iron overload due to thalassaemia when current chelation therapy is inadequate, or SCD or other anemias.
Ferriprox® was previously approved in Canada for the treatment of patients with transfusional iron overload due to thalassemia when current chelation therapy is inadequate in 2015, and subsequently approved for the treatment of iron overload in patients with SCD or other anemias in 2021.
Update: 31 March 2023
No update available.