Reblozyl®
Non-Transfusion-Dependent Thalassaemia
-

Product Information
Scientific name: Luspatercept
Brand name: REBLOZYL®
RESPONSIBLE: Bristol-Myers Squibb -

Clinical Trial/Study Information
Trial Name: BEYOND
Code: NCT03342404
Phase: 2
Eligible patient diagnosis: NTDT
No. of Patients enrolled: 145
Study Sites: 14 Sites per countryCompletion date: November 2022
Scope of the Study / Aim: Reduction of number of transfusions -

Regulatory Information
Status: Authorised
Additional notable points:
- EMA: Approved for the treatment in adult patients of anemia associated with non-transfusion-dependent (NTD) beta thalassemia (2023).
- FDA: N/A
MHRA: Approved for the treatment of adult patients with transfusion-dependent anaemia associated with beta thalassemia (PLGB 15105/0154)
Update: 30 September 2025
Long-term efficacy and safety results from the final analysis of BEYOND spanning an additional 2.2 years of follow up was reported in a publication by Taher A et al. See here.
The results showed a sustained increase of haemoglobin levels for up to approx.. 4.6 years of treatment with luspatercept and improvement of patient-reported tiredness and weakness.
Update: 30 June 2025
Data presented at the 30th EHA Annual Congress (12 – 15 June) in Milan, Italy showed:
Results from real-world settings in:
-
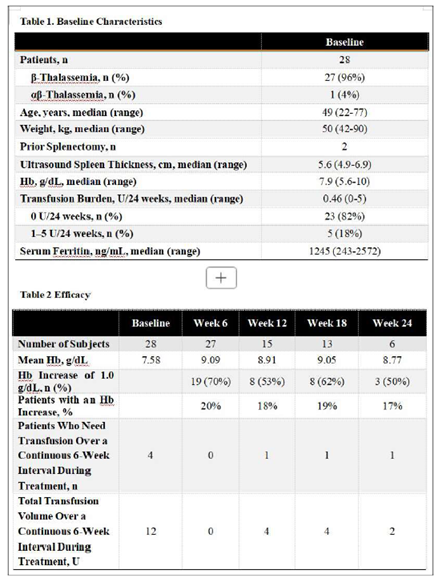
- China: 50% of patients with mild to moderate β-thalassaemia saw a Hb increase of 1g/dL at week 24
- Greece: NTDT patients who had become TDT later in life (median age of becoming TD was 70.5yrs) or had indications requiring transfusions (N=15), became transfusion independent with luspatercept with a mean Hb increase of 1.9g/dL post treatment
Sources: THE IMPACT OF LUSPATERCEPT ON ERYTHROPOIESIS IN A REAL-WORLD COHORT… – BRANCALEONI V – EHA-3090 – Jun 13 2025
REAL WORLD DATA ON IMPROVING ANEMIA WITHOUT TRANSFUSIONS WITH… – Dimopoulou M – EHA-6150 – Jun 14 2025
Update: 31 March 2025
No update available.
Update: 19 December 2024
New data
- Data presented at the 66th ASH Annual Congress (7 – 10 December 2024) in San Diego (USA) showed that:
- Treatment with luspatercept meaningfully improved fatigue-related symptoms reported by patients with NTDT prior to treatment. This was sustained for at least 5 years of treatment.
Update: 30 September 2024
No update available.
Update: 30 June 2024
No update available.
Update: 31 March 2024
No update available.
Update: 20 December 2023
New data
Data presented at the 65th ASH Annual Congress (9 – 12 December 2023) in San Diego (USA) showed that:
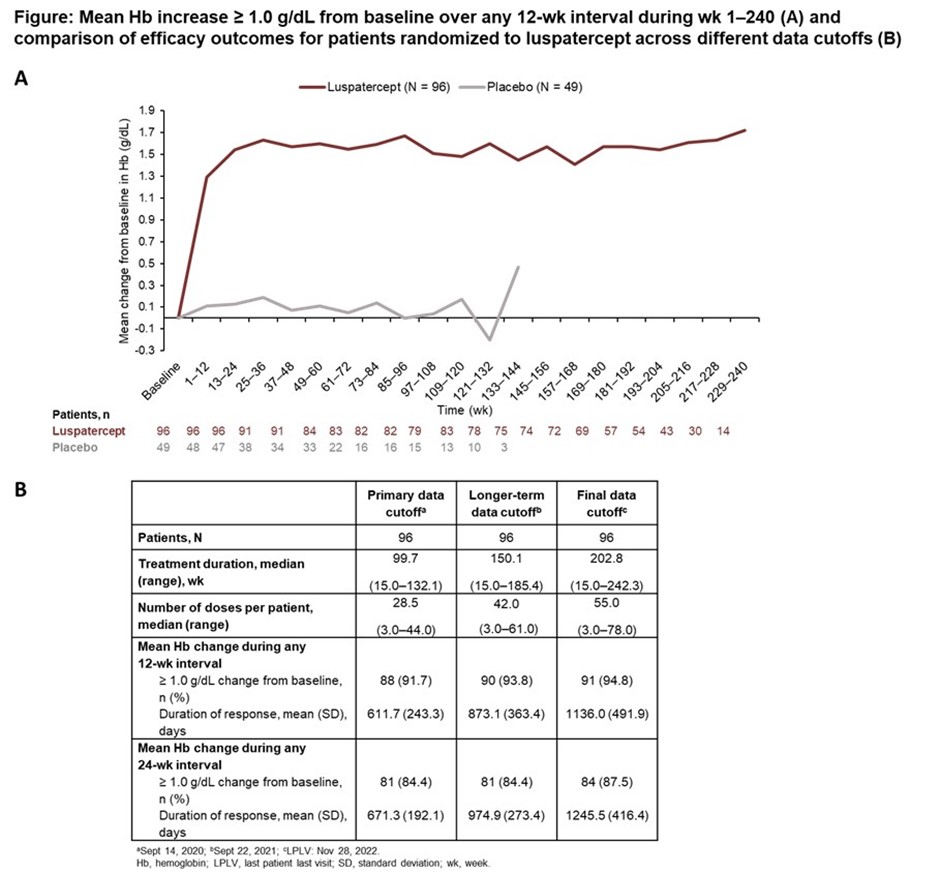
- Final data from the BEYOND Trial were presented.
- Patients had a durable increase in Hb ≥ 1.0 g/dL from baseline (by 12-wk interval) through wk 240 of luspatercept
- In the luspatercept arm, 91/96 (94.8%) pts had a mean Hb increase ≥ 1.0 g/dL from baseline during any 12-wk interval
- The mean total duration of mean Hb increase ≥ 1.0 g/dL from baseline (any 12-wk interval) with luspatercept was 1136.0 days.
Source: https://ash.confex.com/ash/2023/webprogram/Paper174097.html
Update: 30 September 2023
No update available.
Update: 30 June 2023
- Data presented at the 28th EHA Annual Congress (9 – 11 June 2023) in Frankfurt (Germany) showed that patients on luspatercept during the BEYOND trial showed sustained improvement in haemoglobin levels and long-term erythroid response thus requiring fewer transfusions.
Source: https://library.ehaweb.org/eha/2023/eha2023-congress/387973/
Update: 31 March 2023
The European Commission (EC) has granted full Marketing Authorization for Reblozyl® (luspatercept), a first-in-class therapeutic option, for treatment in adult patients of anemia associated with non-transfusion-dependent (NTD) beta thalassemia.
Reblozyl is currently approved in the European Union (EU), United States and Canada to address anemia associated with transfusion-dependent beta thalassemia and 8 transfusion-dependent lower-risk myelodysplastic syndromes. The centralized Marketing Authorization approves use of Reblozyl in all EU member states, as well as Norway, Iceland and Liechtenstein.
The EC approval of Reblozyl was based on results from the Phase 2 BEYOND study, evaluating the efficacy and safety of Reblozyl versus placebo in 145 adults with NTD beta thalassemia. See study results below.
BEYOND Study Results
BEYOND (NCT03342404) is a Phase 2, double-blind, randomized, placebo-controlled, multicenter study to determine the efficacy and safety of luspatercept (ACE-536) versus placebo in adults with non-transfusion-dependent beta thalassemia. The study is divided into the Screening Period, Double-blind Treatment Period (DBTP), Open-label Phase (OLP), and Post-Treatment Follow-up Period (PTFP) and randomized 145 subjects at a 2:1 ratio of Reblozyl versus placebo. All patients were eligible to receive best supportive care, which included red blood cell transfusions; iron-chelating agents; use of antibiotic, antiviral, and antifungal therapy; and/or nutritional support, as needed. The primary endpoint of the study is the proportion of subjects who have an increase from baseline ≥1.0 g/dL in mean of hemoglobin values over a continuous 12-week interval from Week 13 to Week 24 of treatment in the absence of transfusions. Key secondary endpoints include mean change in non-transfusion-dependent beta thalassemia-patient reported outcome (NTDT-PRO) Tiredness and Weakness (TW) domain score and baseline hemoglobin (Hb).
Results demonstrated 74 of 96 (77.1%) patients in the Reblozyl treatment arm achieved the study’s primary endpoint, ≥1.0 g/dL mean Hb increase from baseline, versus 0 of 49 (0%) patients in the placebo arm (P<0.0001).
In a key secondary endpoint of the study, 47 of 96 patients (49.0%) treated with Reblozyl achieved mean Hb increase of ≥1.5 g/dL compared to baseline from Week 37-48 in the absence of transfusions versus 0 patients (0%) in the placebo arm. In the Reblozyl arm, 89.6% of patients remained transfusion free at weeks 1-24 versus 67.3% of patients in the placebo arm. Improvements in patient reported quality of life outcomes (tiredness and weakness) were also observed to correlate with Hb increases.
Serious adverse reactions occurred in 11.5% of patients (n=11) who received Reblozyl and in 25% of patients (n=24) who received placebo. The most common adverse reactions occurring in ≥10% of patients treated with Reblozyl were bone pain (36%), headache (30%), arthralgia (29%), back pain (28%), prehypertension (23%), hypertension (20%), cough (18%), diarrhea (17%), influenza-like illness (17%), asthenia (13%), influenza (13%), insomnia (11%) and nausea (10%).
Sources: https://news.bms.com/news/details/2023/Bristol-Myers-Squibb-Receives-Positive-CHMP-Opinion-for-Reblozyl-luspatercept-for-Adult-Patients-with-Anemia-Associated-Non-Transfusion-Dependent-NTD-Beta-Thalassemia/default.aspx
https://investors.bms.com/iframes/press-releases/press-release-details/2023/Bristol-Myers-Squibb-Receives-European-Commission-Approval-of-Reblozyl-luspatercept-for-Anemia-in-Adult-Patients-with-Non-Transfusion-Dependent-Beta-Thalassemia/default.aspx
Transfusion-Dependent Thalassaemia
-

Product Information
Scientific name: Luspatercept
Brand name: REBLOZYL®
RESPONSIBLE: : Bristol-Myers Squibb -

Clinical Trial/Study Information
Trial Name: BELIEVE
Code: NCT02604433
Phase: 3
Eligible patient diagnosis: TDT
No. of Patients enrolled: 336
Study Sites: 76 Sites per countryCompletion date: 2021
Scope of the Study / Aim: Reduction of number of transfusions -

Regulatory Information
Status: Authorised
Additional notable points:
- EMA: Approved for the treatment of adult patients with transfusion-dependent anaemia associated with beta thalassemia (2020).
- FDA: Approved for the treatment of anemia in adult patients with beta thalassemia who require regular red blood cell (RBC) transfusions (2019).
- MHRA: Approved for the treatment of adult patients with transfusion-dependent anaemia associated with beta thalassemia (PLGB 15105/0154).
Update: 30 September 2025
No update available.
Update: 30 June 2025
Data presented at the 30th EHA Annual Congress (12 – 15 June) in Milan, Italy showed:
Results from real-world settings in:
-
- Turkey: 53.4% of patients showed a good or moderate reduction in transfusion burden
- Italy: Discontinuation of treatment by 62.6% of patients enrolled (N=107) in an early access programme across 19 centres in Italy. The most frequent causes of discontinuation were adverse events and insufficient efficacy (either by the physician or the patient). The median duration of treatment was 378 days.
- Luspatercept treatment increases erythropoietin (EPO) levels and other markers of ineffective erythropoiesis in a real-world setting evaluating 48 patients with TDT in Italy, of whom 17 responded to luspatercept treatment. Levels of HbF increased in those who responded.
- Gene variants involving the TGF-β pathway influence the clinical response to luspatercept. In a real-world cohort, 21 mutated genes were found exclusively in the responders group (16/44 enrolled TDT patients).
Sources: ROLE OF TERTIARY GENETIC MODIFIERS IN RESPONSE TO LUSPATERCEPT… – Leoni S – EHA-4574 – Jun 13 2025|
CLINICAL STUDY ON THE EFFICACY AND SAFETY OF LUSPATERCEPT IN… – Li H – EHA-4776 – Jun 12 2025
CHANGES IN TRANSFUSION BURDEN AND IRON OVERLOAD PARAMETERS IN… – Aydinok Y – EHA-1098 – Jun 14 2025
LUSPATERCEPT UNDER AN ‘ EARLY ACCESS PROGRAM’: AN ITALIAN… – Origa R – EHA-4587 – Jun 14 2025
Update: 31 March 2025
No update available.
Update: 19 December 2024
New data
- Data presented at the 66th ASH Annual Congress (7 – 10 December 2024) in San Diego (USA) from the long-term follow up study (NCT04064060) showed that long-term treatment with luspatercept (median duration >4 years) resulted in decreased serum ferritin levels from baseline (change noted after week 48), stable LIC levels despite lower daily doses of iron chelation therapy (particularly deferasirox). Any changes in LIC levels occurred after week 96. The stabilisation or improvement of iron parameters are linked to the reduction of RBCs transfusion burden and show the long-term benefit of luspatercept treatment.
Update: 30 September 2024
No update available.
Update: 30 June 2024
- Data presented at the 29th EHA Annual Congress (13 – 16 June 2024) in Madrid (Spain) showed that:
- In a real-life setting, TDT patients responded to luspatercept in a similar way as those on the BELIEVE clinical trial i.e. 26% of patients showed a ≥33% reduction of transfusion-burden in weeks 13 – 24 of taking luspatecept.
- Response to luspatercept could be predicted by fetal haemoglobin (HbF) values.
- Reduction in transfusion-burden was found to be more likely in TDT patients who had undergone splenectomy, had lower erythropoietin and higher reticulocyte counts among the participants of the BELIEVE trial.
- Treatment with luspatercept is associated with increased levels of erythropoietin and GDF15.
Sources: Predicting and Evaluating Response to Luspatercept Intransfusion-Dependent Β-Thalassemia: New Insight from a Real-Life Experience.
Characterizing Patterns of Transfusion Burden (Tb) Reduction in Patients (Pts) with Transfusion-Dependent (Td) Βeta-Thalassemia Treated with Luspatercept in the BELIEVE Trial
Analysis of Erythropoiesis and Iron Metabolism in a Cohort of Β-Thalassemia Patients Treated with Luspatercept
Update: 31 March 2024
No update available.
Update: 20 December 2023
New data
- Data presented at the 65th ASH Annual Congress (9 – 12 December 2023) in San Diego (USA) showed that:
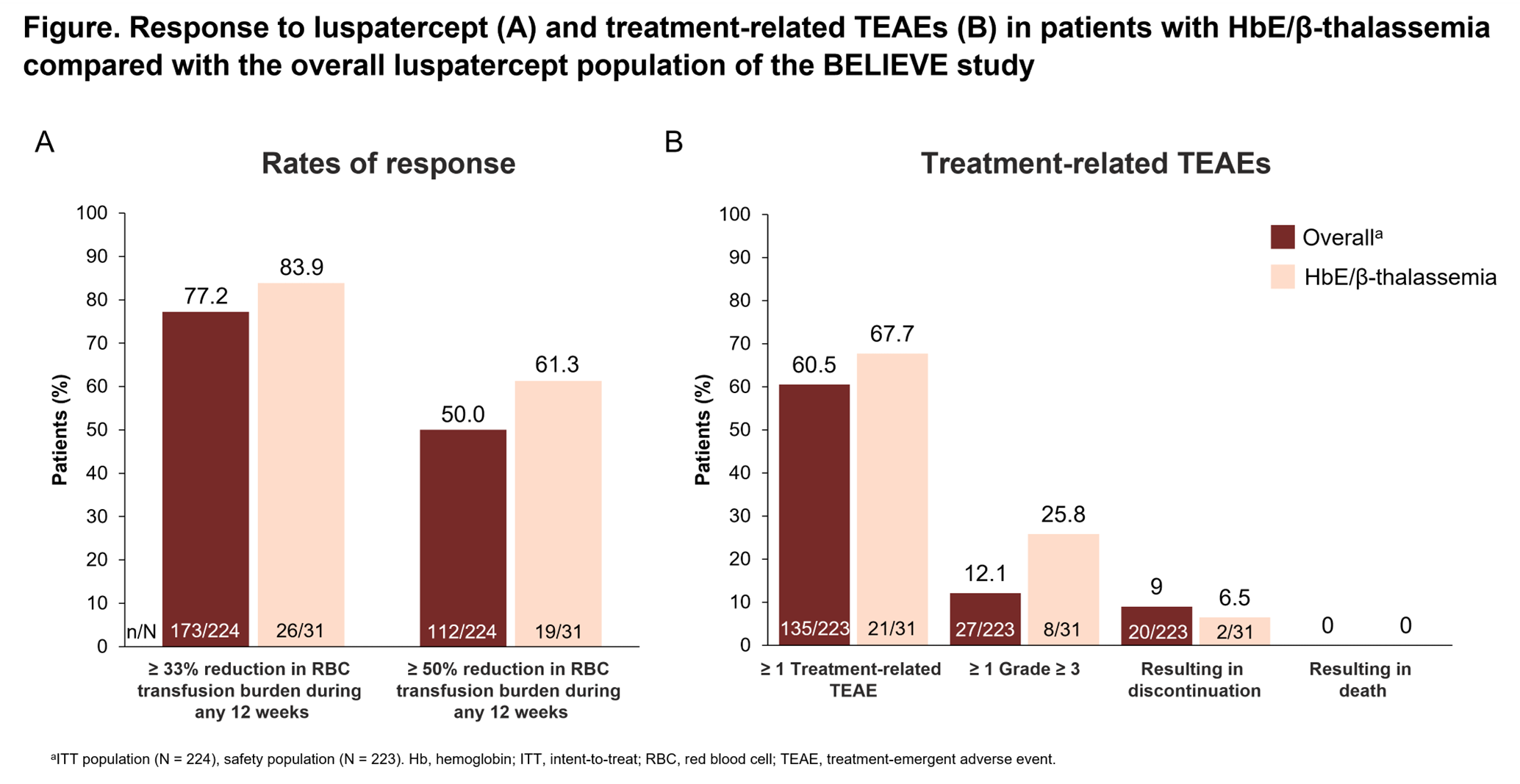
- The HbE/β-thalassemia sub-group of the BELIEVE study had rates of response that were consistent with the larger participant cohort. Furthermore, pts with HbE/β-thalassemia experienced a longer median total duration of response compared with the overall participant cohort with a similar safety profile.
- Findings from the phase 3b long-term follow-up study (LTFU; NCT04064060):
- In the luspatercept group, 180/224 (80.4%) patients had achieved response, continuing the trend showing an increasing proportion of responders with longer duration of treatment observed at previous data cutoffs.
- Liver iron concentration in the luspatercept group was reduced with long-term treatment (mean decrease of 2.80 mg/g dry weight [dw] at week 144)
- Mean changes from baseline in overall serum ferritin levels were −376.27 μg/L for the luspatercept group.
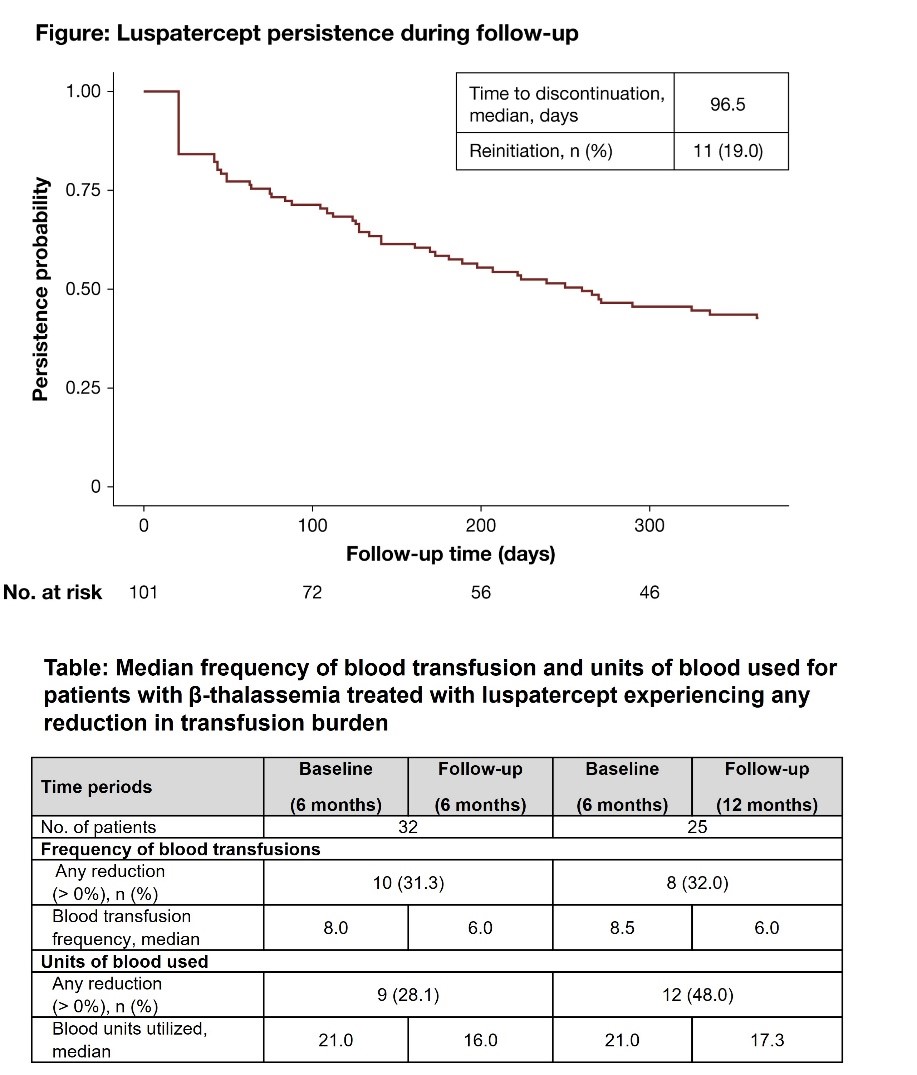
- In the US, a study showed that patients with β-thalassemia treated with luspatercept saw a substantial reduction in transfusion burden, in both the frequency of blood transfusion and units of blood used. As those who remained on luspatercept longer, through 12 months, saw an increased reduction in transfusion burden from baseline, these findings suggest the clinical and real-world benefit of longer-term luspatercept treatment.
Sources: https://ash.confex.com/ash/2023/webprogram/Paper174082.html
https://ash.confex.com/ash/2023/webprogram/Paper174089.html
https://ash.confex.com/ash/2023/webprogram/Paper173378.html
Update: 30 September 2023
No update available.
Update: 30 June 2023
- Data presented at the 28th EHA Annual Congress (9 – 11 June 2023) in Frankfurt (Germany) showed that patients on luspatercept during the BELIEVE trial maintained stable bone health and did not significantly worsen despite reduced transfusions.
Source: https://library.ehaweb.org/eha/2023/eha2023-congress/385914/
Update: 31 March 2023
- The Egyptian Ministry of Health and Population (MoHP), the local healthcare authorities including the Egyptian Drug Authority (EDA) and the Egyptian Authority for Unified Procurement (UPA) have approved the commercialisation of Reblozyl® (luspatercept) in the country.
Reblozyl® (luspatercept) is available in other key markets of the Middle East including Saudi Arabia, UAE, Kuwait and Oman with others in the pipeline.
Pediatric TDT & NTDT
-

Product Information
Scientific name: Luspatercept
Brand name: REBLOZYL®
RESPONSIBLE: : Bristol-Myers Squibb -

Clinical Trial/Study Information
Trial Name: N/A
Code: NCT04143724
Phase: 2a
Eligible patient diagnosis: TDT and NTDT, 6 – 17 years of age
No. of Patients enrolled: 99
Study Sites: 24 Sites per countryCompletion date: July 2027
Scope of the Study / Aim: To evaluate safety and determine the recommended dose in paediatric patients with TDT and NTDT -

Regulatory Information
Status: Not Authorised
Additional notable points:
N/A
Update: 30 September 2025
Part A (patients 12 – 17 years old) of the study nearing completion of enrolment / dosing.
Sources: https://clinicaltrials.gov/study/NCT04143724
Update: 30 June 2025
No update available.
Update: 31 March 2025
No update available.
Update: 19 December 2024
No update available.
Update: 30 September 2024
No update available.
Update: 30 June 2024
The study is staggered and in 2 parts:
- Part A (patients 12 – 17 years old)
- Part B (patients 6 – 12 years old)
Part B will start after the recommendation of the Data Monitoring Committee based on more than 12 months safety data from Part A.
- The safety data is similar to those observed in adults.
- Data presented at the 29th EHA Annual Congress (13 – 16 June 2024) in Madrid (Spain) reported safety data for TDT patients in the dose-finding phase of Part A as follows:
– 12 TDT patients with a median age of 14 years, received luspatercept
– Adverse events were reported in all patients (regardless of dosage – 0.75mg/kg or 1.0mg/kg)
– No adverse event led to luspatercept discontinuation.
α-Thalassaemia
-

Product Information
Scientific name: Luspatercept
Brand name: REBLOZYL®
RESPONSIBLE: : Bristol-Myers Squibb -

Clinical Trial/Study Information
Trial Name: N/A
Code: NCT05664737
Phase: 2
Eligible patient diagnosis: α-thalassaemia HbH disease, either TD or NTD over 12 years of age
No. of Patients enrolled: 249
Study Sites: 36 Sites per country
Expected completion date: June 2026
Scope of the Study / Aim: To determine the efficacy and safety of luspatercept in adult patients (over the age of 18) and evaluate safety and pharmacokinetics in adolescent patients (between age 12 – 18) with α-thalassaemia haemoglobin H -

Regulatory Information
Status: Not Authorised
Additional notable points:
N/A
Update: 30 September 2025
No update available.
Update: 30 June 2025
No update available.
Update: 31 March 2025
No update available.
Update: 19 December 2024
A global phase 2 multicentre study is now underway to study luspatercept in adults and adolescents with α-thalassaemia HbH disease. The study will evaluate safety and efficacy in adults and safety and pharmacokinetics in adolescents.
Both transfusion dependent and non-transfusion dependent patients can participate, provided they fulfil the eligibility criteria.